SRI scientists partner with Mapp Biopharmaceutical to accelerate FDA approval for clinical trials.
THE CHALLENGE
Ebola virus (EBOV) causes severe, frequently fatal illness in humans, characterized by hemorrhage, multi-organ failure and a shock-like syndrome. The 2014 EBOV outbreak in West Africa resulted in approximately 30,000 infections and 10,000 deaths. A number of health care providers were infected and were brought to the United States for treatment, resulting in subsequent transmission to health care workers within the U.S. There are no approved vaccines or treatments for EBOV infection.
THE SOLUTION
ZMappTM, manufactured by Mapp Biopharmaceutical, Inc. (San Diego, CA), is a cocktail of three monoclonal antibodies (c13C6+c2G4+c4G7; see Figure below) targeted to the surface EBOV glycoprotein. This cocktail has shown protection against EBOV in preclinical models. While efficacy has been clearly demonstrated, little was known about the safety and pharmacokinetics of ZMapp, and this information would be required before initiation of human clinical trials.
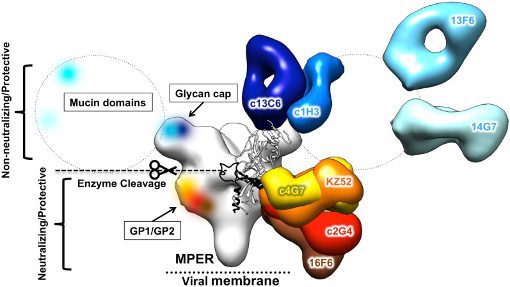
Background:
SRI holds the contract for preclinical development of anti-infective agents through the Division of Microbiology and Infectious Disease (DMID) in the National Institute of Allergy and Infectious Diseases (NIAID). NIAID, Mapp, and SRI partnered to conduct safety studies of ZMapp, and to develop appropriate methods for measuring the three antibodies in the blood following treatment.
SRI designed a rodent toxicity study to determine the safety of ZMapp prior to initiation of human clinical trials. The study, conducted under FDA’s Good Laboratory Practice (GLP) regulations, was intended to be the first of several safety studies performed; however, during the study, the 2014 EBOV outbreak resulted in several individuals within the U.S. contracting EBOV. With a high probability of fatality, and no other treatment options available, FDA approved treatment of humans under expanded access provisions (EAP; “compassionate use”) prior to its formal approval for human clinical testing.
How it works:
Because of the critical need to use ZMapp, and the realization that the cocktail’s use in human cases was imminent, SRI, in consultation with NIAID, Mapp and the FDA, redesigned the ongoing toxicity study to include additional dose administrations and to extend the post-treatment recovery period for extended evaluation of responses. We also developed an ELISA-based detection method in serum, and developed assays to determine the presence of anti-drug antibodies that could potentially inactivate the ZMapp antibodies. Due to the urgency of the outbreak, including the possibility that EBOV infections could spread throughout the U.S., SRI placed a very high priority on completing all of the necessary work in record time.
SRI modified the design of the ongoing toxicity study and completed the final report within eight weeks of completing the experimental phase of the study. The study demonstrated that while ZMapp had minor effects on the liver, these resolved quickly after cessation of treatment, and the three-mAb cocktail was very well tolerated. SRI also developed methods for measuring the three antibodies in blood, and demonstrated that anti-drug antibodies were not formed against the cocktail components.
Validation:
Based on these results, Mapp filed an Investigational New Drug (IND) application for ZMapp in February 2015, only seven months after initiation of the safety program; the industry average for this development period is normally about three years. SRI continued to work with ZMapp, developing additional ELISA methods for measuring antibodies in multiple species including human, and conducting studies to identify potential cross-reactivity of the cocktail in human and animal tissues.
Partner with us:
SRI Biosciences partners with clients to seamlessly integrate basic research, drug discovery, and nonclinical and clinical development. Our turnkey and customized services include models for research and drug discovery; identifying targets and mechanisms of action; efficacy; pharmacology and pharmacokinetics; safety; and clinical research studies.
For additional information, please contact SRI Biosciences at biosciences@sri.com
Research and development activities described were supported by:
National Institute of Allergy and Infectious Diseases (NIAID), Division of Microbiology and Infectious Diseases (DMID), of the National Institutes of Health (NIH) under Contract Number HHSN272201100022I. [P20750]
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.